
Christmas is over and now it’s time to head back to studying, researching and working! That daunting early morning alarm that you haven’t heard for weeks, cold and wet mornings combined with spending too much time on my first day back in the freezers is not something I want to be doing to try and get back into the swing of things – but how should I go about it? Ease myself back in? Or throw myself back in? Definitely slow and steady this year. So, I thought I would show you a slow day in the life of a PhD student – the first day back after Christmas.

8:20am – Definitely did not want to get up this morning! So it was a much later start than usual, plus it’s always that little bit easier getting out of bed if there is light coming in through the window.

8:55am – Packing time! Making sure I have all my new stationary supplies, diary, laptop, thesis and most importantly, lunch!

9:15am – Sitting at my desk which seems like quite a surreal feeling considering I’ve had nearly a month off with Christmas and hospital etc. But I do like a routine, so I will be glad to be back doing something productive rather than not being able to move a lot like I was before Christmas. First job – unpack, organise desk and check emails! Luckily I have been checking them on odd days over the holidays so I didn’t have as many to deal with this morning as I would have done.
10:00am – Due to my sickness before Christmas, I was way behind on my lab book. Luckily I had made quick notes of what I did on each day and what my fellow lab mates had done for me, so next job was to decipher them and get my lab book up to date with all the experiments I did and the samples I collected. Then the more tricky job was to find where the samples were that others had collected for me.


11:34am – FOUND THEM! It was much easier than I was anticipating thanks to an organised past me and helpful lab mates who had left me notes saying where they had left my samples. So, I popped them on some ice to thaw so I could extract the protein from them and do a Bradford assay which I have shown you on a previous day in the life of a PhD student chapter.

12:31pm – Another main priority I had today was to get some cells out of the freezer and get them growing so I could start doing some experiments again. Our lab tech was preparing the stem cell stocks that the whole lab use, so today I took some of the cancer stem cells out that I and a few others use. So after running round the lab turning everything back on again and letting it warm up, I got some cancer stem cells out of the liquid nitrogen stores and transported on some dry ice just before thawing. I took it upon myself to pour some water over the dry ice (after my cells had been taken out!) to take a much cooler picture rather than just a vial in a box full of ice! Yawn! This looks so much more exciting!

12:41pm – The key to thawing cells is to do it as quickly as possible! My cancer stem cells were frozen down in a freezing solution before Christmas before storage – but if the cells are in that solution and not frozen, they don’t like it and it will start to kill them off. So, I have a few minutes to get my cells from -80C to 37C and get them out of the freezing solution! The vial of cells is put into the water bath until the cells have thawed. We then mix them with some more media and spin that mixture in a centrifuge that spins it 1500 times per minute!! After a few minutes, there is a pellet of cells at the bottom of my tube and the liquid sat on top. That liquid contains the freezing solution so we pour that off and then mix the cell pellet with some more media before putting it into one of our flask to let them grow. Hopefully I did it quick enough! I will find out tomorrow when I check on my cells – if they are all stuck down in the flask it means I did a good job at freezing them and unfreezing them quickly. If there are lots of cells floating – it means a lot of cells have died and maybe I was too slow in the freezing or thawing. But the good news is that these are cancer stem cells and in comparison to the embryonic stem cells, they are really easy to grow!
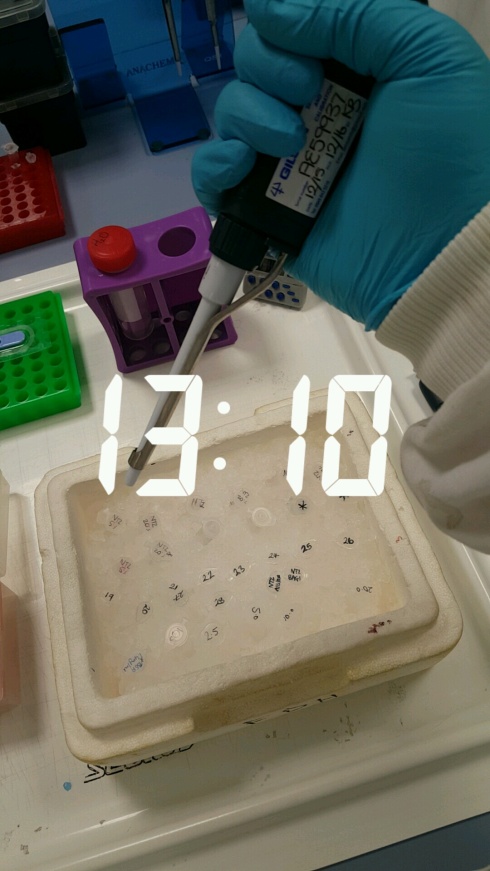
1:10pm – Time to extract my protein from my samples! Feels very weird holding a pipette in my hand for after so long! Luckily I haven’t forgotten how! We lyse the cells or break them up using a process called sonication. As we do this in the water bath, there are high frequency vibrations that agitate the cells and cause them to break up releasing the protein locked inside! Much like my cells were mixed in with the freezing solution, my protein is mixed in here too – so I need to spin my samples in the centrifuge again but this time they need to be spun about 10,000 times per minute!! However, this time I do still get a pellet at the bottom of my tube surrounded by some liquid, but this time we want to get rid of the pellet and keep the surrounding solution. It is different this time compared to using my cells is because of the speed the centrifuge and the size of the thing we are interested in. So, our cells are quite big in the grand scheme of things and so will pellet at the bottom of the tube even at slower speeds. Cell debris, which is what we are getting here when we isolate the protein from our samples, is smaller than whole cells, so you need to spin the centrifuge even faster. However, proteins are very small! So the speed we have been spinning at is not fast enough to force the proteins to the bottom of the tube to make a pellet, so they float around in the liquid still. We would need speeds much much faster than 10,000 a minute to force the proteins into a pellet!

1:52pm – time for another Bradford assay, but my first Bradford assay of 2017 🙂 I’ve talked you through this technique before in a previous chapter here, but in short I have just extracted the protein from my samples, so this allows me to estimate how much protein is in my samples. And from the dark blue colour in all the wells on the right, it means there is lots of protein in my samples 🙂

2:09pm – Need to get back into the habit of keeping my lab book up to date now. So I have got all the data from today and processed it, so making sure I write about my Bradford assay and cells in detail!! and before I forget what I did!

2:22pm – One of my science New Years resolutions was a challenge to read 230 papers this year – one for each working day. So – the first day back and the first paper. I work on embryonic stem cells and am about to start looking at chromatin state and methylation so I thought this would be useful for me and the new stage I’m about to start (as soon as the cells are up and running! :/)

3:01pm – After neglecting my thesis all Christmas and my transfer viva on Friday, it is probably time that I give this a read. I’ve got some more data since submitting my thesis so I have all my new figures ready, just need to read through and get prepared for any questions I may get asked. Very anxious about this – so need to get more prepared!

5:07pm – 2 hours of viva prep done but still don’t feel like I have achieved much and don’t know enough. So I’m off home to continue reading and hopefully some of the information will be retained. Fingers crossed! But should probably get back to it! Head down and prep, prep, prep!
.
Definitely a nice, slow and easy day to get back into the swing of things after the holidays. How was everyone’s first day back at work? Did you achieve much? I imagine everyone is back at work by now, but what are your tips for easing back into your routine after the festive period?
S.x
Please don’t forget to keep up to date on all my new blog posts and more! Find me and Soph talks Science on Facebook, Twitter and Instagram. Make it your New Year’s Resolution.
